Our Research
The Schmidt lab aims to build in vitro human 2D and 3D models of the developing cerebellum that will allow us to study neural stem cell trajectories, cellular signaling cascades and gene expression dynamics in healthy and diseased states. Particularly we study pediatric cancers of the brain in order to clarify mechanisms of tumor initiation and to identify therapeutic vulnerabilities.
Most pediatric brain cancers arise sporadically, caused by disruptions of developmental cell signaling pathways and transcriptional cascades. Our lab combines human neural stem cell cultures, brain organoids and patient-derived mouse xenografts with emerging genomic technologies to establish accurate pediatric brain cancer models. By studying the origin of neural pediatric cancers during the development of the brain, the Schmidt lab will clarify cell types and gene expression dynamics leading to tumor formation.
Our research focuses on medulloblastoma, a cancer of the hindbrain and the most common brain cancer in children. We are particularly interested in developing faithful human models for Group 4 medulloblastoma, the most common yet least understood medulloblastoma subgroup.
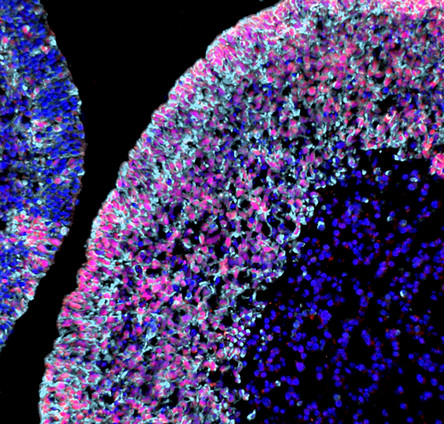
Cerebellar Organoid
Unipolar brush cells as the cell-of-origin for Group 4 medulloblastoma
The cellular origin of Group 4 medulloblastoma has been linked to the proliferative subventricular zone of the human rhombic lip in the embryonic hindbrain, the birthplace of unipolar brush cells. Using transcription factor genetic reprogramming approaches in neural stem cells our lab aims to develop in vitro cell cultures of human unipolar brush cells. Our goal is to study unipolar brush cell biology, their cell-of-origin for Group 4 medulloblastoma, and identify cancer driver genes and therapeutic targets.
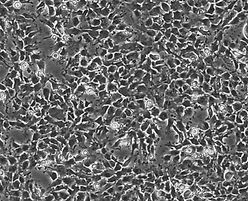
Neuroepithelial Stem Cells
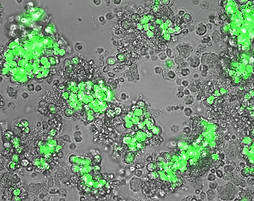
Medulloblastoma PDX Cell Line
Uncovering genetic drivers and therapeutic vulnerabilities of Group 4 medulloblastoma using cerebellar and patient-derived xenograft organoids
While the rhombic lip in humans splits into a ventricular and subventricular zone, the full extent of this compartmentalization cannot be found in mice and macaques, emphasizing the need for human G4MB models. Our lab is using cerebellar organoids and organoid co-culture techniques in combination with gene targeting and gene reporter tools to study the cell-of-origin and physical space of G4MB origin in the human embryonal hindbrain. The use of patient-derived xenograft organoid co-cultures will allow us to establish reliable Group 4 medulloblastoma cultures from primary tumor samples and study individual patient tumor biology.
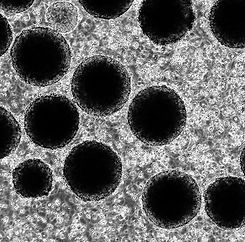
Cerebellar Organoid Cultures
PDX Organoid Co-Culture
Inducible Gene-Reporter Organoids
Oncogenic mechanism and therapeutic targets of PRDM6-amplified medulloblastoma
High expression or amplification of PRDM6 is the most common genetic driver in Group 4 medulloblastoma occurring in >20% of cases. In the healthy brain, expression of PRDM6 is restricted to early embryonic development, but its function in the immature hindbrain is unknown. We have previously shown that forced expression of PRDM6 in human iPSC-derived hindbrain neuroepithelial stem cells generates medulloblastoma at low penetrance after orthotopic transplantation in mice (Schmidt et al., Sci Rep 2024, PMID 38992221). To further elucidate the role of PRDM6 in medulloblastoma formation we are employing a combination of genome-wide sequencing techniques such as ATAC-seq, CUT&RUN sequencing and RNA-seq in various PRDM6-amplified tumor models.
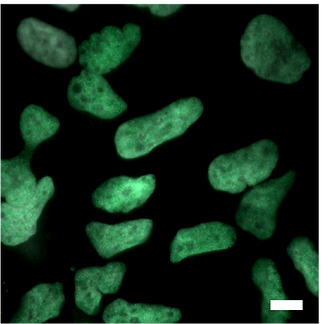
PRDM6-V5 Immunofluorescence
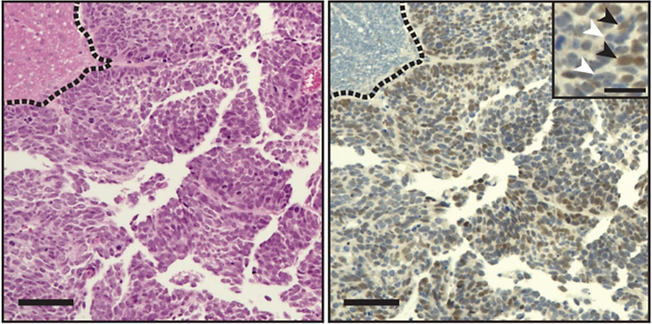
Hematoxylin Eosin and V5 Immunocytochemistry in PRDM6-NES tumors